Bronchial Thermoplasty for Refractory Asthma – A Blessing In Disguise

Asthma is a chronic health problem and affects 17 million people in our country. It is a disease characterized by airway inflammation, hyper responsiveness, bronchoconstriction and mucous hypersecretion. Till date, the main stay for asthma therapy had been inhaled corticosteroids and inhaled bronchodilators. In addition, leukotriene antagonists (montelukast) and phosphodiesterase inhibitors are also used. Though a majority of asthmatics improve with these conventional therapies, there is a subset of severe asthma patients who fail to respond despite maximal doses of inhaler medications. This subset constitutes 5 to 10% of the asthma population and these people have persistent symptoms, poor quality of life and frequent severe exacerbations. Earlier, these patients were initiated on oral corticosteroids which when used for longer durations lead to several side effects. It is in these people that the newer therapy“Bronchial thermoplasty” is indicated.
Bronchial thermoplasty (BT) – Principle and Technique:
Bronchial thermoplasty (Alair system; Boston Scientific) is a bronchoscopic intervention which works by ablating the airway smooth muscle by applying controlled radio frequency energy via a dedicated catheter (Figure 1). When thermal energy (65 C) is applied to the airway wall, the smooth muscle mass is reduced and this results in improvement in airway lumen diameter as well as a decrease in the bronchoconstriction. The procedure is performed under short general anaesthesia, in three sessions, each one three weeks apart. Each session targets one part of the lung (first session – right lower lobe; second session – left lower lobe and the third session- both upper lobes). During the session, an adult therapeutic bronchoscope is passed into the airway and the thermoplasty catheter is advanced through the working channel of the scope. All the visualized segments and the sub-segments of the target lobe are sequentially targeted. Each session lasts 45 to 60 minutes and around 60 – 100 activations of the BT probe are typically applied per session.
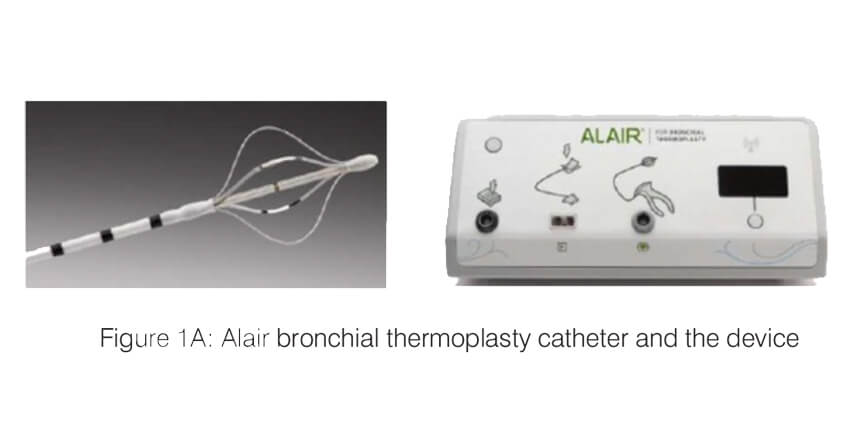
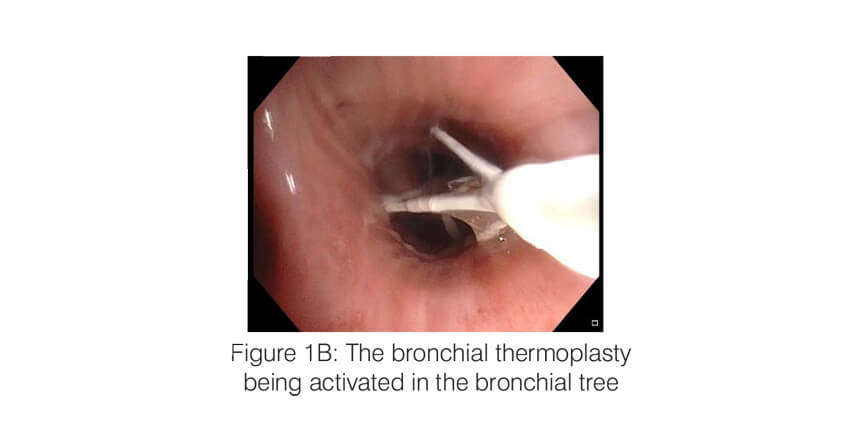
Bronchial thermoplasty – Evidence for use:
Several randomized controlled trials have been performed to evaluate the clinical effectiveness and long term safety of BT. Three such trials are the AIR trial, RISA trial, and the AIR 2 trials. All these included patients with moderate to severe asthma. All the trials showed that there was a significant improvement in the quality of life scores, patient symptoms, rescue medication use and exacerbation rates. In the AIR 2 trial, which is the largest RCT of 300 patients, there was an 84% reduction in the emergency visits and 73% reduction in
hospitalizations for asthma exacerbations. All the effects remain stable for up to five years. Five years follow up studies have shown that there are no long term side effects of the procedure.
Bronchial thermoplasty – Yashoda Hospitals, Somajiguda experience:
Over the last four months, six patients with severe refractory asthma successfully underwent the procedure at our center, Yashoda Hospitals, Somajiguda. A detailed description of the first patient and the summary of the remaining are presented here.
Baseline characteristics of the patients:
Among the six patients who underwent bronchial thermoplasty, the majority were women (n=5). The ages ranged from 30 years to 57 years. All of them had long-standing asthma (Table 1). All the patients were using high dose inhaled corticosteroids, long-acting beta-agonists and montelukast regularly despite which the asthma was uncontrolled. Two patients (Case 3 and Case 5) were also on regular oral corticosteroids. Case 1 also tried Omalizumab for one year but without relief. All the patients had frequent exacerbations (mean no. of exacerbations – 6/year) and three patients required ICU admission for an asthma exacerbation in the last year.
Table 1: Baseline clinical characteristics of the patients treated with bronchial thermoplasty
| Bariatric surgery in India | General ward | Private room |
|---|---|---|
| gastric bypass surgery cost | ||
| gastric band surgery cost | ||
| gastric sleeve surgery cost | 4,75,000 | |
| roux-en-y gastric bypass surgery cost | ||
| the gastric balloon surgery cost | ||
| laparoscopic gastric bypass surgery cost |
Table 2: Baseline lung function and quality of life scores of the patients treated with bronchial thermoplasty
| Surgery | General ward | Private room |
|---|---|---|
| Hernia operation cost – open surgery | 100,000 | 1,75,000 |
| Laparoscopic hernia surgery cost | 1,35,000 | 1,75,000 |
All six patients had an obstructive pattern on spirometry (Table 2). Post-bronchodilator FEV1 was less than 60% predicted in all but one patient (Case 6). All of them had impaired quality of life and poor symptom control. Most of them used reliever medicines daily in addition to their controlled medicines. GINA assessment of asthma control was poor in all the patients. Asthma Quality of Life Questionnaire (AQLQ) score was low in all the patients. Lower the Asthma QOL score, poorer is the QOL score. Asthma Control Test (ACT) score was calculated at the baseline in all the patients and it was <19 in all the patients depicting poor asthma control. Symptom control was also poor in all the patients as assessed by the Asthma Control Questionnaire (ACQ 6).
Safety and tolerability of bronchial thermoplasty:
All the patients underwent three sessions of thermoplasty three weeks apart. The procedure was well tolerated in all the patients and there were no intraprocedural complications. Among the 18 sessions, there was a mild worsening of symptoms after four sessions.
Two patients required admission for infective exacerbation in between the sessions. Both improved with antibiotics and did not require oxygen support or ICU care.
Clinical improvement after bronchial thermoplasty:
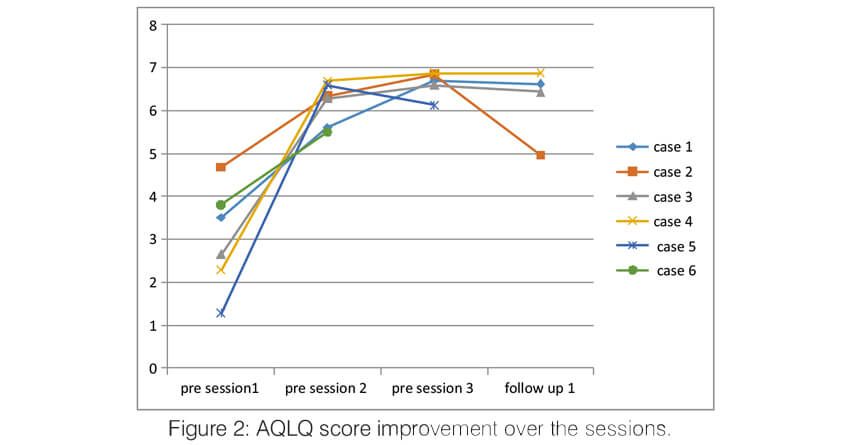
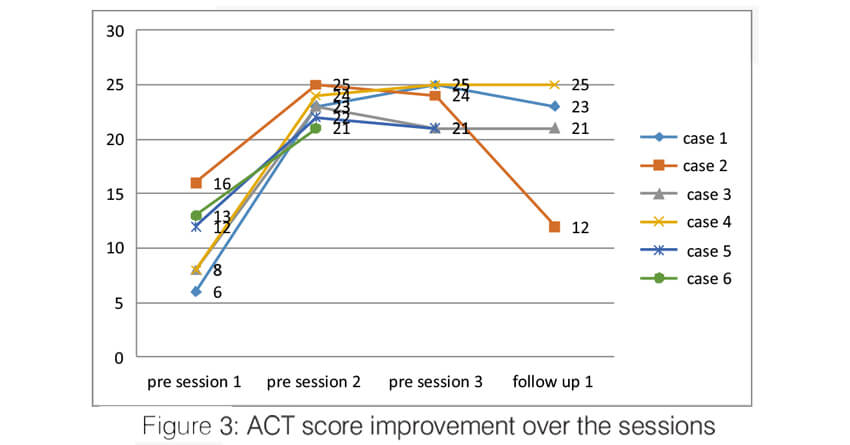
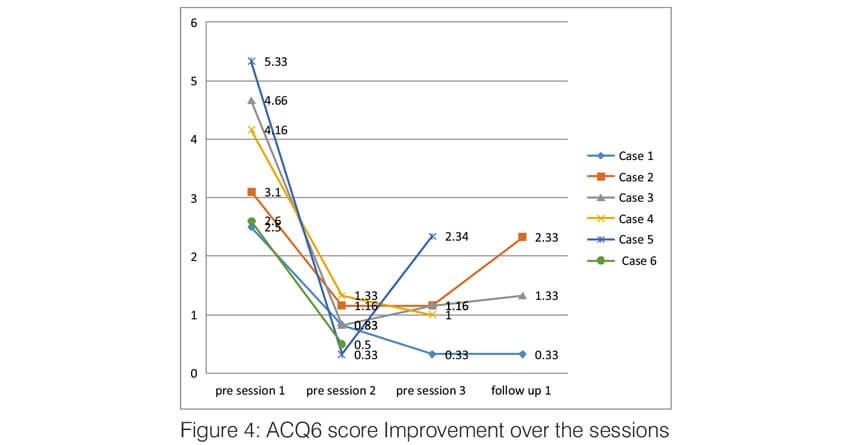
Over the sessions, there was a significant improvement in the quality of life of the patients. The AQLQ score significantly improved in all the patients (Figure 2). All of them had an improvement of >0.5 points which is the minimal but clinically important difference (MCID). Asthma control significantly improved in all the patients. Asthma control test score improved in all the patients to >19 (well controlled) and remained the same until the last follow up (Figure 3). The Asthma Control Questionnaire 6 score also significantly improved in all. The ACQ 6 score (Figure 4) decreased by > 3 points in all the patients (MCID of 0.5). During the follow-up, two patients (case 2 and case 5) had a mild exacerbation requiring oral steroids one month after follow up.
The lung function (measured by post-bronchodilator FEV1) improved in two patients and remained stable in the remaining four patients. This is consistent with the results of the clinical trials which showed that the lung function remains stable post bronchial thermoplasty. Of the two patients who were on oral corticosteroids, we were able to completely stop the OCS in one patient (Case 3) and reduce the dose by >50% in the other patient (case 5).
We also performed endobronchial biopsies pre and post thermoplasty (3 weeks). Histopathologic examination of the biopsies revealed a decrease in the smooth muscle mass, a decrease in the inflammatory cells as well as a decrease in the mucosal goblet cells following the thermoplasty.
Summary:
Bronchial thermoplasty is a very promising treatment for patients who are refractory to the current therapies of asthma. In such patients, the following benefits were noted
- Significant improvement in the Quality of life
- Significant improvement in the asthma control
- Improvementinthepatient’ssymptomsandrescue medicine use
- Improved / stable lung function
- Decrease in the exacerbations
- Decrease in the airway smooth muscle mass, airway wall inflammation and mucosal goblet cells.
Though there was a mild increase in symptoms transiently following the procedure in some patients, the benefits shall definitely out weight the risk of the procedure. There is a strong need to increase the awareness of this procedure both amongst the clinicians and the patients
Case vignette (patient 1):
A 53-year-old lady, homemaker, a known asthmatic since 35 years presented with progressively worsening symptoms over the past 5 years. She was already using high doses of inhaled corticosteroids, inhaled bronchodilators, montelukast and fexofenadine. In view of elevated serum IgE levels, she was started on therapy with biologics (omalizumab) in 2016, but there was no good response and was stopped after a year. She had 5 exacerbations in the last one year, two of which were managed with oral steroids and three exacerbations with oral steroids and antibiotics. From January 2017, she had dyspnea grade 3 (mMRC), cough with expectoration, frequent nocturnal awakenings and her room air saturations were 93-94% with the significant limitation of daily activities. Her lung function testing showed severe obstruction, post-bronchodilator FEV1/FVC and FEV1 were 53.8 (65%) and 0.91L (45%) respectively. Her asthma was uncontrolled as per GINA assessment (4/4). Quality of life was poor (ACT score-6, ACQ6 score-2.5, AQLQ score -3.6).
The patient was taken up for bronchial thermoplasty. Three sessions were done and there were no peri-procedural complications. Over the sessions, there was a significant improvement in her lung function. FEV1/FVC improved from 53.8(65%) to 71.1(86%) and FEV1 from 0.91L (45%) to 1.59L (77%). Quality of life has also improved very significantly as assessed by ACT (25), ACQ6 (0.33) and AQLQ (6.7), and asthma was well controlled as per GINA assessment. Her oxygen saturation improved to 97% on room air. She has no reliever medication use and is able to do all her daily activities without any functional limitation. Histopathological examination of endobronchial biopsies before and after BT showed a significant decrease in the ASM, inflammatory cells and submucosal goblet cells post procedure. She had now completed three months follow up and continues to remain asymptomatic with no rescue medicine use and no exacerbations.
About Author –
Dr. V Nagarjuna Maturu, Consultant Pulmonology, Yashoda Hospital, Hyderabad.
MD, DM (Pulmonology)
His special interest lies in Bronchoscopy and Endobronchial Ultrasonography (EBUS), Medical thoracoscopy, Sleep Medicine, Rigid bronchoscopy and Therapeutic bronchoscopic procedures.






 Appointment
Appointment WhatsApp
WhatsApp Call
Call More
More

